PMMA & Science
Polymethyl Methacrylate: Acrylic
Polymethyl methacrylate (PMMA) belongs to a family of synthetic polymers.
It is better known as acrylic and is sold under brand names such as Akrylon®, Altuglas®, Perspex®, Plazcryl®, Plexiglas®, Oroglas®, Solarkote™ Diakon® , Acrypet™, Crylon® and Crylux®.
PMMA is a tough, highly transparent material with excellent resistance to ultraviolet radiation and weathering. It can be coloured, moulded, cut, drilled, and formed. These properties make it ideal for many applications including airplane windshields, skylights, automobile taillights, and outdoor signs.
Like all plastics, acrylics are polymers. The word, polymer, comes from the Greek words poly, meaning many, and meros, meaning a part. A polymer, therefore, is a material made up of many molecules, or parts, linked together like a chain. Polymers may have hundreds, or even thousands, of molecules linked together. More importantly, a polymer is a material that has properties entirely different than its component parts.
Methyl methacrylate
What is it made of?
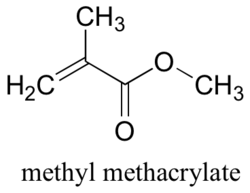
During polymerisation, one leg of this double bond breaks and links up with the middle carbon atom of another methyl methacrylate molecule to start a chain. This process repeats itself until the final polymer is formed.
Methyl methacrylate may be formed in several ways. One common way is to react acetone [CH3COCH3] with hydrogen cyanide [HCN] to produce acetone cyanhydrin [(CH3)2C(OH)CN]. This in turn is reacted with methyl alcohol [CH3OH] to produce methyl methacrylate.
Other similar monomers such as methyl acrylate [CH2=CHCOOCH] and acrylonitrile [CH2=CHCN] can be joined with methyl methacrylate to form different acrylic plastics. When two or more monomers are joined together, the result is known as a copolymer. Just as with methyl methacrylate, both monomers have a double bond on the middle carbon atoms that splits during polymerisation to link with the carbon atoms of other molecules. Controlling the proportion of these other monomers produces changes in elasticity and other properties in the resulting plastic.
